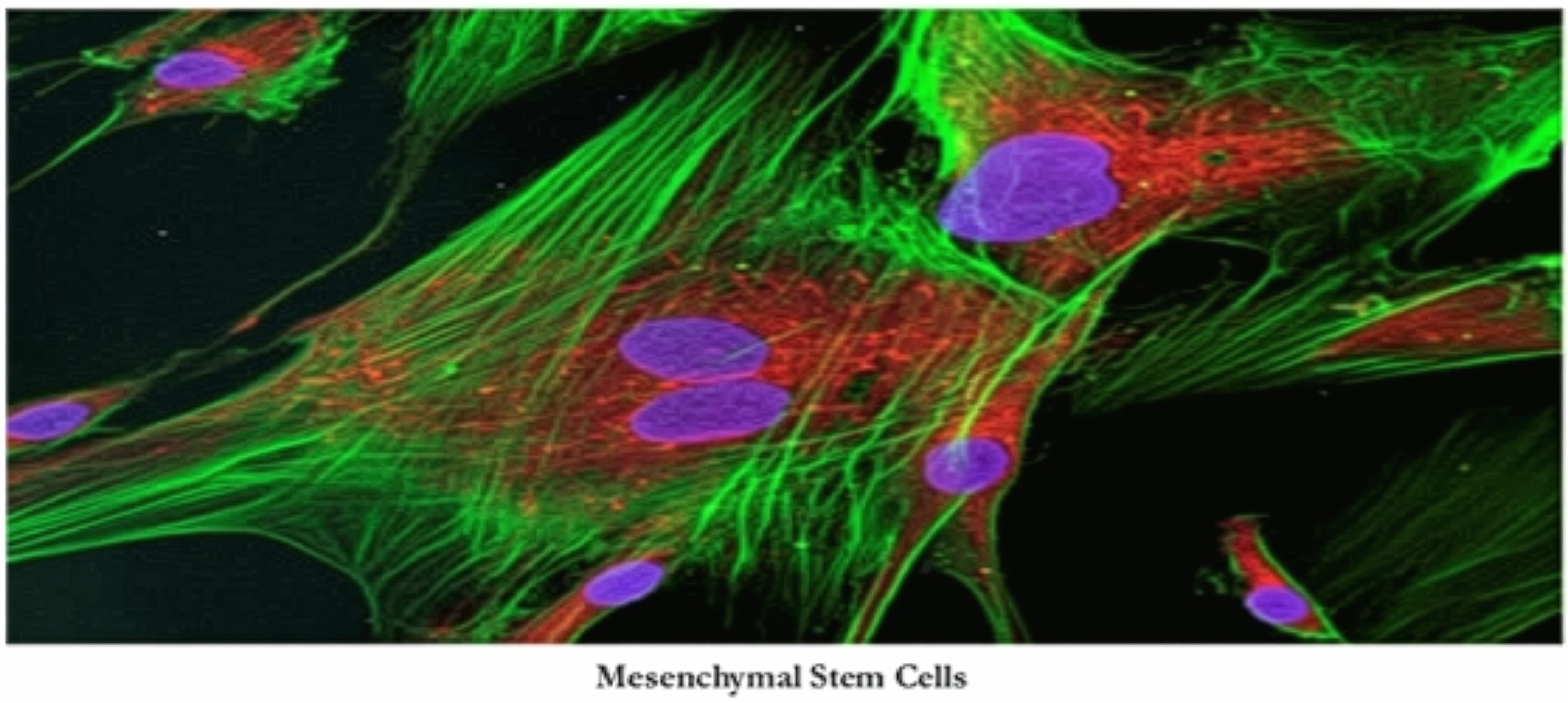
Osiris obtained the composition of matter patent on the MSC and characterized it in a Science paper that has been cited more than any other in the history of medicine.
This is a result of over 1,700 clinical studies registered with Clinical Trials.gov, covering 1,338 conditions, a scope unprecedented in the pharmaceutical industry.
As the leader in cellular regeneration, Osiris brought the first approved MSC drug to market in the U.S. and also obtained the first regulatory approval for an MSC drug.
Other products became standards of care, including Osteocel, the first product in the U.S. market containing MSCs, and Grafix – with a 71% healing rate for non-healing diabetic foot ulcers, the leading cause of amputation.
Osiris sold Osteocel for $50 million, sold its MSC portfolio to Mesoblast for $100 million in cash, equity, and royalties, before the firm was acquired by Smith and Nephews for $660 million. Most importantly, the firm’s products have saved thousands of lives.